Safety Alert: Recall of Ground Turmeric Powder From Lieber’s and Other Brands

Ground turmeric powder from Lieber’s, and other kosher brands, are being recalled, the Food and Drug Administration has announced.
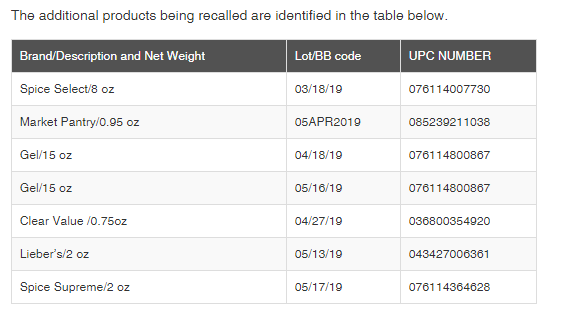
Last month, the FDA announced that Gel Spice, Inc. was recalling Fresh Finds Ground Turmeric Powder because the product contains elevated lead levels. This week, Gel Spice expanded its recall of ground turmeric powder to include additional brands, including Lieber’s, Spice Select, and others (see table below for full list of recalled products).
Routine sampling by New York State Department of Agriculture and Markets’ food inspectors and subsequent analysis of the product by the New York State Food Laboratory had revealed the elevated level of lead. No illnesses have been reported to date in connection with this problem.
According to the FDA, lead can accumulate in the body over time, and too much can cause health problems, including delayed mental and physical development and learning deficiencies. Expectant women, infants and young children especially should avoid exposure to lead. People concerned about blood lead levels should contact their physician or health clinic to ask about testing.

Consumers with questions about the recalled product may call (201) 564-0435.
The FDA’s recall page for this product is http://www.fda.gov/Safety/Recalls/ucm515328.htm
To Read The Full Story
Are you already a subscriber?
Click "Sign In" to log in!

Become a Web Subscriber
Click “Subscribe” below to begin the process of becoming a new subscriber.

Become a Print + Web Subscriber
Click “Subscribe” below to begin the process of becoming a new subscriber.

Renew Print + Web Subscription
Click “Renew Subscription” below to begin the process of renewing your subscription.



